Abstract
Background
Myeloma outcomes have improved due to the use of novel agents. However, patients who have failed bortezomib and lenalidomide represent a significant challenge. In New Zealand, myeloma patients are initially treated with CyBorD, transplant (if eligible) and VTD consolidation. Lenalidomide/dexamethasone is approved as third line therapy, or as second-line in patients with neuropathy. Compassionate access of carfilzomib became available in May 2016 for patients who have progressed after bortezomib and lenalidomide.
Methods
Patients with myeloma treated with a carfilzomib triplet were identified from our database. After two initial doses of carfilzomib at 20mg/m2 patients were given a fixed dose of 60mg as a 30 minute infusion. Carfilzomib was given on Days 1, 2, 8, 9, 15 and 16 of a 28-day cycle. To streamline administration, carfilzomib was infused via a Portacath in most patients. Patients received dexamethasone 20mg PO on days of carfilzomib (1, 2, 8, 9, 15 and 16), and cyclophosphamide 300mg/m2 PO Days 1, 8, and 15, thalidomide 100mg daily, lenalidomide 25mg Days 1-21 or venetoclax 800mg daily. Routine support care was valaciclovir, cotrimoxazole and allopurinol. Aspirin 100mg was given daily to reduce cardiovascular events. A baseline echocardiogram was obtained in all patients. Treatments were continued for 9 cycles, followed by maintenance (reducing carfilzomib to only Days 1 and 2 of a 28-day cycle, but continuing oral therapy) until treatment failure. Patients who progressed by IMWG criteria continued treatment if they continued to derive benefit. Patients were followed with monthly myeloma blood monitoring but did not routinely have bone marrow assessments to assess CR status. All statistical analysis was done using IBM SPSS version 20.
Results
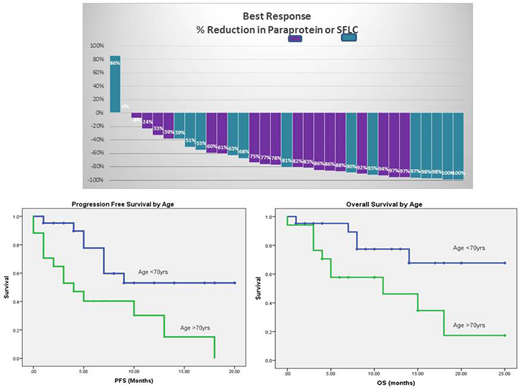
Between 18/5/16 and 21/5/18, 38 patients with RRMM were treated with a triplet containing carfilzomib, dexamethasone and cyclophosphamide( KCd n=33), lenalidomide (KRd n=3) thalidomide (KTd n=1), or venetoclax (KVd n=1). All patients except one, received at least 2 cycles of treatment and 19 currently continue therapy. The median number of cycles given is 10 (range 2-27). The median age at treatment initiation was 68yrs (range 51yrs to 91yrs); there were 17 patients >70yrs and three >80yrs. Patients had a median of 3 prior therapies and 18pts had received a ABMT, Prior treatment included bortezomib (100%), thalidomide (92%), lenalidomide (95%), and pomalidomide (18%). Carfilzomib triplets were generally well tolerated. One patient discontinued due to shortness of breath after two cycles. One patient had a non-STEMI cardiac event prior to routine use of aspirin, but continued on KCd. The best response was ≥CR 8% (n=3), ≥VGPR 32% (n=12), ≥PR 74% (n=28) ≥ MR 84% (n=32), four patients had SD and two progressed without benefit.
Of the 38 patients, 21 have progressed with a median PFS of 9 ± 2.4 months. PFS was better in patients aged <70yrs, 53% at 18 months vs 0% for those aged >70 (median 4 months)(p=0.008). So far 15pts have died with a median OS of 18 ± 3 months. All deaths were in patients who had progressed. Outcomes were superior in those aged <70 yrs, OS 68% at 18 months (median OS not reached), versus those aged >70 yrs, OS 17% at 18 months (Median OS 11±6 months)(p=0.008) The median survival from relapse was poor at 2 months. However, of the 6 patients alive after progression, 3 received an anti-CD38 antibody as salvage and 3 remain on treatment with KCd, the longest continues on treatment at 15 months.
Summary
Fixed dose carfilzomib triplets are effective salvage therapy in myeloma patients who fail bortezomib and lenalidomide therapy. Treatment is well tolerated, even in elderly patients, albeit with inferior outcomes.
Simpson:Abbvie: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Acerta: Research Funding; Merck: Honoraria, Research Funding; BeiGene: Research Funding; Sanofi: Research Funding; Bristol-Myers Squibb: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Celgene: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Novartis: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; MSD: Honoraria; Amgen: Research Funding, TRAVEL, ACCOMMODATIONS, EXPENSES; Janssen: Honoraria, Research Funding. Chan:Amgen: Honoraria; Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal